
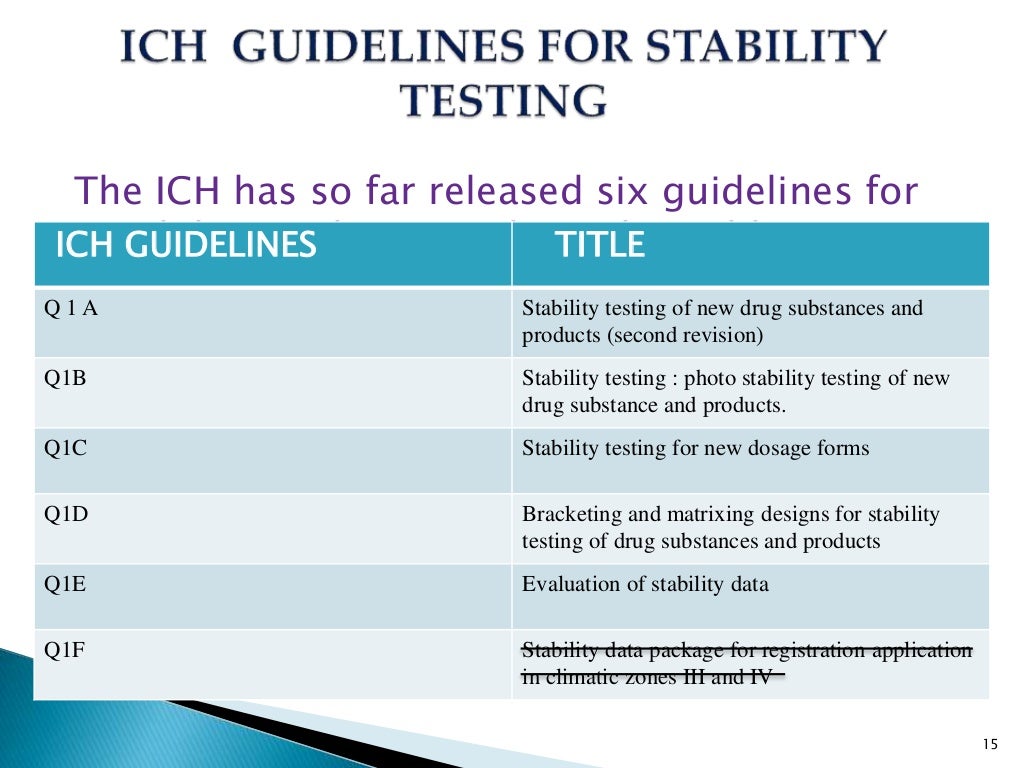
1993 (available from ICH Secretariat, c/o IFPMA, 30 rue de St-Jean. 6.1. Stability testing of new drug substances and products. The 12 modules included in the course are based on ICH GCP Principles and. Any amendment (s) should also bear the amendment number (s) and date (s). well as incorporating the recent modifications made to the GCP guidelines. The guidance should be read in conjunction with other relevant ICH guidance. 6.1.1 Protocol title, protocol identifying number, and date. (i) The alternative procedure (s) or course (s) of treatment that may be available to the subject, and their important potential benefits and risks. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is unique in bringing together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceuticals and develop ICH guidelines.
Ich guidance how to#
Such prospective evaluation is necessary to justify any revision of the present recommendations of the ICH guideline S1 (EMA 2016). This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ICH guidance Q1A(R2) Stability Testing of New Drug Substances and. Sponsors may choose onsite monitoring, centralized monitoring, or a combination of both. These hypotheses stimulated ICH to test in an ongoing common exercise by Drug Regulatory Agencies and Industry if there are chances to reduce the number of rodent bioassays.
Ich guidance update#
The guidelines that reached Step 4 include an update to the questions and answers document for ICH’s M8. ICH updated on the progress of four of its guidelines, three that reached Step 4 of the ICH process in recent months and one that reached Step 2. Quality Tolerance Limits (QTLs) should be established and upon detecting deviations from the limits that were pre-defined, it should be evaluated to decide if action is required. The new additions bring the total for ICH members to 17 and the number of observers to 32.


Ich guidance trial#
Sponsors should implement quality systems to manage quality throughout the trial process (design, protocol, tools, procedures, data collection, as well as the collection of info that impacts decision making).

The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is a project that brings together the regulatory authorities of Europe, Japan and the United States and experts from the pharmaceutical industry in the three regions to discuss scientific and technical aspects.


 0 kommentar(er)
0 kommentar(er)
